مخطط الموضوع
Information about the course
Institute of Natural and Life Sciences
Department of Biotechnology
Course: Introduction to Biotechnology
licence Program Title: BiotechnologySemester: S03
Course Unit Title: Fundamental Teaching Unit 01
Number of hours of personal work for the student: 67,5 hours
Number of credits: 6
Course Coefficient: 3
VHS: 15 weeks
Evaluation Method: 40% Continuous Assessment, 60% Final Exam
sunday: course
wednesday: course + Directed Study
Course : Dr. SAHLI MOHAMED
Directed Study: melle LITIM SARA
Course Description
Course Description: Introduction to Biotechnology
This introductory course provides a comprehensive overview of the field of biotechnology, covering its fundamental concepts, principles, and applications. Students will explore the various biotechnological techniques and tools used in areas such as genetic engineering, molecular biology, microbiology. The course will also examine the ethical, legal, and social implications of biotechnological advancements.
Key Topics:
- Basics of genetics, molecular biology, and microbiology
- Overview of biotechnological tools and techniques.
- Applications of biotechnology in agriculture, healthcare, and environmental sustainability
- Biotechnology in medicine: from drug development to gene therapy
- Ethical, environmental, and societal issues related to biotechnology
- Current trends and emerging areas in the field.
The course is designed to give students a foundational understanding of biotechnology and prepare them for more specialized studies in the field.
Learning Outcomes: By the end of the course, students will be able to:
- Understand the basic principles of biotechnology and its applications in various industries.
- Discuss the ethical and societal challenges associated with biotechnological innovations.
- Demonstrate a fundamental knowledge of biotechnological methods and their practical uses.
Course Objectives
Course Objectives: Introduction to Biotechnology
Understand the Fundamentals of Biotechnology:
Gain a solid understanding of the basic principles of biotechnology.Learn the Tools and Techniques:
Become familiar with the major biotechnological tools and techniques used in the field of biotechnology.Explore Biotechnology Applications:
Explore the diverse applications of biotechnology in various sectors, including agriculture, healthcare, environmental management, and industrial biotechnology.Examine Ethical and Societal Implications:
Analyze the ethical, legal, and social issues related to biotechnology, including concerns about genetic modification, environmental impact, and biotechnology regulation.Understand the Role of Biotechnology in Medicine:
Understand how biotechnology is revolutionizing medicine, with applications in drug development, diagnostics.Recognize Current Trends and Future Directions:
Stay updated on cutting-edge developments in biotechnology, such as the future potential of biotechnology in addressing global challenges.Develop Critical Thinking and Problem-Solving Skills:
Encourage students to think critically about biotechnological advances and how they can be used to solve real-world problems, while considering the broader impacts on society and the environment.Foster Interdisciplinary Understanding:
Promote an interdisciplinary approach to biotechnology, integrating knowledge from biology, chemistry, engineering, and ethics to better understand the complexity of biotechnological systems.
Course Content
- Introduction.
1.1. The Origins of Biotechnology.
2. Biotechnology Applied to Environmental Issues.
1.2. Evolution of Biotechnology Over Time.
1.3. Current Major Issues in Biotechnology and Bio-nanotechnology.
1.4. Definitions of Green, White, and Red Biotechnologies.
1.5. Typical Products of Biotechnology.
1.6. Relevant Industrial Sectors.
1.7. Challenges in Biotechnological Innovation.
2.1. Climate Change and Ecosystem Evolution.
2.2. Management of Microbial, Plant, and Animal Resources.
2.3. Agro-environmental Pollution (Water, Air, Soil).
3. Biotechnology in Agronomy for Food Purposes.
3.1. Biotransformation and Preservation.
3.2. Production of Food Matrices in Bioreactors.
3.3. Food Safety, Traceability, and Quality.
4. Biotechnology and Industry for Non-Food Purposes.
4.1. Bioenergy.
4.2. Biomaterials and Agro-polymers.
4.3. Biomolecules and Cellular Activities.
5. Microbial Biotechnology and Infectiology.
5.1. Diagnostics.
5.2. New Therapeutic Pathways.
5.3. Combatting Doping and the Use of Narcotics.Chapter 01: Introduction to Biotechnology
chapter 01
Introduction to Biotechnology
Introduction
Biotechnologies encompass all techniques that utilize the resources of living organisms (tissues, cells, proteins, etc.) or their components (genes, enzymes, etc.), whether recombined or not, to generate knowledge, goods, or "bioservices" (services based on biotechnology, such as the production of proteins or transgenic animals).
Biotechnologies can be defined as "all technological applications that utilize biological systems, living organisms, or their derived parts to create or modify products or processes for specific uses." The use of biological systems for product manufacturing has been known since ancient civilizations. Evidence has been found of knowledge regarding the crossbreeding of animal species and plants to better meet specific needs, dating back to ancient Egypt.
1.1. The Origins of Biotechnology
About 10,000 years ago, humans began modifying the living world around them to improve their daily lives by selecting the plant and animal species they needed, sowing their crops, and breeding their livestock.
They also observed and utilized the fermentation processes caused by microorganisms, yeasts, and bacteria, of which they were, of course, unaware at the time. They discovered that raw materials could be transformed in various ways:
- Sugar could be converted into alcohol and carbon dioxide, forming the basis for the production of bread, wine, and beer;
- Alcohol could be converted into acetic acid during vinegar production;
- Bacteria could multiply in milk to turn it into yogurt.
Often discovered by chance, beer, wine, cheese, and other products emerged at different points in human civilization, several millennia before Christ.
1.2. Evolution of Biotechnology Over Time
In the 19th century, the work of three scientists laid the foundations for modern biotechnology. Louis Pasteur and Robert Koch developed bacteriology and the concepts of microbial disease, immunity, and vaccination. Johann Gregor Mendel described the laws governing the transmission of biological traits between generations (laws of heredity).



Louis Pasteur Robert Koch Johann Grégor Mendel
The second half of the 20th century saw a significant acceleration in the understanding of living organisms, thanks to advances in science and technology. In 1944, American microbiologist Oswald Avery demonstrated that DNA is the carrier of heredity. In 1953, James Watson and Francis Crick built upon Avery's work by discovering the structure of DNA, enabling the direct manipulation of genetic traits.

James Watson et Francis Crick
Gene insertion techniques for "foreign" genes into the genetic material of bacteria were first developed in the 1970s, marking a decisive step in the biotechnology revolution.
The first product of modern biotechnology was recombinant insulin, a protein produced by the pancreas that helps regulate blood glucose levels, essential for diabetic patients who can no longer produce their own insulin. In 1978, the human insulin gene was transferred into the bacterium Escherichia coli at Herbert Boyer's lab at the University of California, San Francisco.
Since the mid-1990s, the field of transgenesis has received significant media attention and continues to expand, with expected advancements in nanotechnology and bioinformatics.
1.3. The Major Current Challenges of Biotechnology and Bio-Nanotechnology.
-Biotechnology is multidisciplinary in nature, involving contributions from: engineering, computer science, cellular and molecular biology, microbiology, genetics, physiology, biochemistry, immunology, virology, recombinant DNA technology, and more.
-Today, it is estimated that over 20% of new drugs launched worldwide come directly from biotechnology, and 80% of drugs currently in development are based on biotechnological discoveries or tools. However, biotechnology is not limited to human health; challenges for animal health, agri-food, industry, and the environment are also significant.
-Transgenic biotechnology is now radically new: it relies on knowledge that was not even taught before.
-The economic prospects are substantial. How can we finance long and costly developments?
-These technologies often evolve faster than our understanding of their impacts, making it difficult to assess the benefits of a new application and the risks it entails.
1.4. Definition of Red, White, and Green Biotechnologies
Biotechnologies can be classified into three categories: red, white, and green.
Red biotechnology pertains to medical processes, such as designing organisms to produce antibiotics or developing gene therapies through genome manipulation. This field impacts health, particularly in the pharmaceutical industry, where much current research relies on biotechnology.
White biotechnology (also known as gray biotechnology) relates to industrial processes, such as developing living organisms for chemical production. It is generally less resource-intensive than traditional industrial processes. Initial applications are found in sectors like polymers, fuels, solvents, construction, textiles, and various chemical products.
Green biotechnology refers to applications in agriculture, such as developing transgenic plants that can grow under specific environmental conditions with or without certain chemicals. Green biotechnology includes all in vitro and laboratory interventions on the embryos, organs, tissues, cells, or DNA of plants to either control or accelerate their production or modify their characteristics. Researchers hope to address agricultural challenges while ensuring food production, energy production, and biomaterial production in an environmentally sustainable manner.
1.5. Typical Products of Biotechnology
GMOs
Genetically modified organisms (GMOs) are products of green biotechnology. They offer certain advantages, such as simplified cultivation practices and reduced pesticide application.Medicines and Vaccines
Historically, medicines (excluding vaccines and serums) were produced by the chemical industry. Since the 1980s, biotechnology has been used to produce treatments that are impossible or too costly to obtain through chemical synthesis. The first applications involved the production of vaccines and antibiotics. Biotechnology allows for the replacement of products extracted from human or animal organs with molecules produced through genetic engineering.
Example:- Insulin produced by bacteria instead of extracted from pig pancreases for diabetes treatment.
Cloning
Therapeutic cloning involves creating human organs or tissues from cells for therapeutic purposes.Example:
In cases of myocardial infarction, cardiac muscle cells can be created to replace damaged heart cells.
Biobased Materials (BBM)
These are substances or molecules produced naturally or through biological processes. Their origins can be bacterial, plant, or animal. These materials can include:- Fibers: Spider silk, collagen, bacterial cellulose for dressings, artificial skin, or implants.
- Gels: Latex, polysaccharides for ophthalmic lenses, dressings, and artificial skin.
- "Massive" materials: Coral, mother-of-pearl, ceramics for prosthetics and bone substitutes.
In Food
*Production of genetically modified foods or animals:Improvement of Existing Products:
- Seedless grapes and citrus fruits.
- Development of fruits and vegetables with high antioxidant levels.
*Creation of New Product:
- Identification of the active components in milk and the associated genes, followed by the modification of the genetic makeup of dairy cows so that they produce more of these components in their milk. One of the applications: milk with coagulation factor for hemophiliacs.
Animal Feed: Improvement of the nutritional qualities of plants, obtaining lignin-poor crops: to enhance livestock health, making it easier for ruminants to digest or chew.
- Functional Foods: The production of functional foods (incorporation of a nutrient intended for healing) through GMOs is more economical than traditional production using bacteria. Example: Development of a banana variety for the production of multiple proteins aimed at immunizing children against bacteria and viruses causing diarrhea.
- The Environment: Environmental biotechnology focuses on environmental preservation, improvement of energy processes, and raw materials (new processes, pollution control, energy efficiency, alternative energy). Example: Production of biofuels: Made from biomass, these biofuels offer two advantages:
- A reduction in pollutant gas emissions (which impact health, vegetation, buildings, and the greenhouse effect)
- The preservation of non-renewable fossil natural resources. Currently, two types of biofuels can be blended with fossil fuels:
- Alcohols and their derivatives (methanol, ethanol).
- Esters and their derivatives (FAME). Comparative example: Ethanol production In a complete cycle—production, transfer, and consumption:
- 1 liter of gasoline = 3.1 kg of CO2
- 1 liter of ethanol (chemical production) = 2.3 kg of CO2
- 1 liter of ethanol (biotechnological production) = 15 g of CO2
Bioremediation: Bioremediation involves the biodegradation or transformation of polluted areas contaminated by heavy metals, explosives, oil, or toxic chemicals. Selected and genetically modified plant varieties can capture and accumulate heavy metals from the soil, such as arsenic. Modified microbes residing in the soil can digest certain contaminants, converting them into harmless substances.
1.6. Affected Industrial Sectors:
Companies utilize industrial biotechnology to:
- Reduce costs,
- Increase profits,
- Enhance product quality,
- Optimize processes and their monitoring,
- Improve technology safety and hygiene,
- Comply with environmental regulations.
Recombinant DNA and Genetic Engineering: Molecular biology has led to the most significant discovery in biotechnology: it is now possible to isolate the gene responsible for coding the production of certain substances, transfer it into another host organism, and thus produce useful proteins more efficiently. Thanks to these advancements, biotechnology now produces hormones, vaccines, blood clotting factors, and enzymes on a large scale. Additionally, the biotechnological production of proteins avoids the drawbacks of production from higher organisms:
- Unlike the culture of microorganisms, large-scale culture of higher organism cells is impractical due to slow growth and frequent contamination.
- The cost of cell culture is significantly higher than that of microbial culture.
- The source of cells from higher organisms is much more limited compared to unicellular organisms, which also reproduce easily and quickly.
Fermentation: Along with biocatalysis, fermentation processes are among the oldest forms of biotechnology. Fermentation is the application of microbial metabolism to transform a material into value-added products. This process can produce an incredible variety of useful substances, such as citric acid, antibiotics, biopolymers, and single-cell proteins, among others. The potential is immense and vast; it simply requires knowing the appropriate microorganism, controlling its metabolism and growth, and being able to utilize it on a large scale.
Fuels and Organic Products as Alternatives to Oil: Oil is a non-renewable raw material, meaning that its uncontrolled or increasing use is limited. Biotechnology, on the other hand, uses renewable materials, allowing for its controlled use to potentially extend indefinitely. In the event of oil depletion, biotechnology can provide two solutions: new fuels and an alternative source of organic products. For example, utilizing waste from sugarcane production to obtain alcohol is a process that leads to energy savings.
Biocatalysts:
Application of Enzymes in the Industrial Sector:
Enzymes are becoming increasingly important in sustainable industrial development. They have already been employed in the development of industrial processes to produce waste-free products or those containing minimal biodegradable waste. Indeed, enzymes can replace toxic or corrosive chemicals in several processes. Moreover, their advantage lies in their ability to be used, deactivated, and broken down into simpler, completely biodegradable products.
Many industrial processes operate at high temperatures or pressures, or under highly acidic or basic conditions. Enzymes can avoid these extreme conditions as well as the use of corrosive reagents.
Here is a quick overview of enzymatic processes currently used in various sectors to reduce chemical load by eliminating the industrial production of aggressive, toxic, or simply polluting substances:
Detergent Industry:
- Enzymatic degradation of proteins, starch, and grease stains in laundry,
- Use of lipolytic enzymes in dishwasher detergents,
- Use of enzymes as surfactants.
Textile Industry:
- Stone washing of denim fabrics,
- Enzymatic desizing of flat-woven cotton fabric,
- Eco-friendly bleaching,
- Enzymatic degreasing of cotton fabrics,
- Degumming of silk.
Starch Industry: Enzymatic production of dextrose, fructose, and special syrups for baking, confectionery, and beverage industries.
Beer Industry: Enzymatic degradation of starch, proteins, and glucans from the grain mixture used in beer production.
Bakery and Bread Industry: Enzymatic modification of carbohydrates and proteins in grains to improve bread properties.
Wine and Juice Industry: Enzymatic degradation of pectin from fruits in the production of juices and wines.
Alcohol Industry: Degradation of starch into sugars prior to fermentation and alcohol production.
Food and Additives Industry:
- Improvement of nutritional and functional properties of animal and plant proteins,
- Conversion of lactose from milk and whey into sweeter and more easily digestible sugars,
- Production of cheese flavors.
Animal Feed Industry: Enzymatic hydrolysis of protein material from slaughterhouses to produce high-nutritional value meals for animal feed.
Cosmetic Industry: Biotechnological production of collagen and other products for use in beauty creams.
Paper Industry:
- Enzymatic dissolution of pitches,
- Eco-friendly bleaching of pulp,
- Enzymatic control of the viscosity of starch coatings.
Tanning Industry: Preparation of hides and removal of hair and fat.
Oils and Fats Industry: Enzymatic hydrolysis of fats and lecithin, and synthesis of esters.
Fine Chemical Industry: Synthesis of organic substances.
1.7. Challenges of Biotechnological Innovation.
Research and Development: The primary declared challenge of research on GMOs pertains to both food and health. Chemical and biological treatments have improved agricultural production conditions over the years; however, the use of phytosanitary products (herbicides, insecticides, fungicides) has rendered this human activity polluting. In the health sector, research focuses on diagnostics, treatments, and the development of vaccine products.
- Socio-economic Factors: The potential economic benefits of employing genetic engineering are numerous and possibly substantial. However, given that transgenic plants have only been cultivated since 1995 and in a limited number of countries, there is still insufficient data to empirically confirm or refute these potential benefits.
- Geopolitical Factors: The Earth is home to approximately six billion individuals today, a number that is rapidly increasing, with significant disparities between populations based on geographic regions.
- Ethical Questions: Biotechnologies raise significant and challenging ethical questions. An extensive international discourse is underway regarding these issues. These ethical considerations primarily relate to the very nature of genetic research and its applications, particularly concerning human health: it involves living organisms and our genetic heritage, which gradually reveals its history, richness, complexity, potentialities, and predispositions.
- Research Transfer Weakness: The historical weakness in transferring scientific research to technological innovations has been evident in the biotechnology sector, characterized by a low level of research involvement in the economic valorization of discoveries. This has resulted in a scarcity of "biotech" companies, which are founded on the initial exploitation of scientific innovations for technological purposes. The few notable exceptions have only highlighted the overall poverty of the sector. However, in recent years, the situation has begun to change.
Chapter 02 : Biotechnologies Applied to Environmental Issues
Chapter 02
2. Biotechnologies Applied to Environmental Issues
2.1. Climate Change and Ecosystem Evolution
Climate change and its potential impacts are now well recognized by major global powers; however, their economic, social, and political institutions have been slow to respond. There is a clear and urgent need for these entities to accelerate and unify their efforts to reduce greenhouse gas emissions and adapt population behaviors to climate change.
To address this, it is essential to:
Increase yields on already cultivated lands.
The enhancement of crop yields is imperative for two primary reasons:
• New lands available for cultivation are scarce, and farmers bear the responsibility of not encroaching upon forests or biodiversity-rich areas, which are also the primary existing carbon sinks.
• The availability of arable land is highly uneven across continents, and due to increasing urbanization, there has been a significant decrease in the amount of arable land available per capita: Between 1960 and 2000, the amount of arable land per capita decreased by 40%. In 1960, there were 4.3 hectares of arable land per person; by 2000, this had dropped to 2.2 hectares per person, and by 2020, it further declined to 1.8 hectares per person.
Currently, the yields of cereal crops are stagnating in Northern countries. Researchers agree that 90% of yield increases will stem from a better understanding of plant biology, improved agronomic practices, and increased utilization of genetics (characterization and potential modification of genomes).
2.1.1. Reduction of Greenhouse Gas Emissions (GHG):
Agriculture currently accounts for 14% of GHG emissions. However, it would have required cultivating between 860 and 1500 million hectares more than in 1960 to achieve current production levels without technological changes. Intensive agriculture, characterized by the adoption of selected seeds, the use of pesticides and fertilizers, irrigation, and mechanization, has increased yields and thus preserved land. Contrary to common belief, it has contributed to a reduction in GHG emissions per ton harvested. Genetically modified organisms (GMOs), by enhancing the performance of existing varieties, further contribute to this reduction in GHG emissions per ton of food produced.
2.1.2. Utility of Herbicide-Tolerant Crops:
A study conducted in Brazil indicates that between 1996 and 2000, the cultivation of genetically modified soybean, corn, and cotton reduced the use of water, fuel, inputs, and CO2 emissions. Specifically, herbicide-tolerant soybean, combined with no-till or reduced tillage techniques, has increased the productivity of this crop by decreasing labor time and reducing the cost of insecticide treatments by 24 to 32%. It has also facilitated double cropping within a year, using both winter and summer varieties.
2.1.3. Upcoming Introduction of Drought-Tolerant Corn:
Numerous research projects on drought tolerance are currently underway worldwide. To maintain yields under water stress conditions, seed companies are employing multiple, often combined, research approaches. The most studied plant is corn, with the first two drought-tolerant varieties expected to be commercialized soon.
2.2. Management of Microbiological, Plant, and Animal Resources:
Until now, collections of organisms (microbial, plant, animal, and human cells) and their components (tissue fragments, nucleic acids, proteins, etc.) were dispersed across various facilities: research centers, laboratories, or hospitals. Accessing these resources was challenging, outcomes were uncertain, and their utilization was not controlled. Therefore, it is essential to consolidate these collections and their organisms within Biological Resource Centers responsible for acquiring, validating, studying, and distributing them. To establish these operations under optimal conditions, four parameters must be considered:
- Scientific Rigor: Research and study of gene networks involved in cellular function and dysfunction require biological resources with guaranteed origin and quality.
- Safety: The diversity and uncontrolled emergence of collections can pose risks to health and the environment (e.g., dissemination of pathogens).
- Ethical Requirements: While there exists a legislative and regulatory framework for the scientific use of collections, it is not fully enforced (particularly regarding human-derived biological resources).
- Economic Regulation: Currently, there is evidence of uncontrolled exchanges and irreversible losses. Specific standards for access to biological collections would promote scientific development and rational industrial applications.
Biological Resource Centers have evolved into strategic infrastructures for biotechnology. Ensuring quality and traceability is essential, especially considering the vast opportunities presented by genome analysis and post-genomic studies: identification of genes of interest, modeling, diagnostic and therapeutic applications, biodiversity, and emerging diseases.
To ensure compliance with all procedures, Biological Resource Centers that handle human biological resources will adhere to a forthcoming ethical charter. This charter regulates the origin of samples and corresponding information, processing, transformation, storage, distribution, and/or transfer of biological samples, intellectual property and valorization, as well as the relationships between different Biological Resource Centers.2.3. Agro-environmental Pollution (Water, Air, Soil)
2.3.1. Bioremediation
It is important to recall that bioremediation is the application of biotechnology to the treatment and reuse of waste products. Let’s examine some applications in this field. Biological purifiers are a good example of simple applied biotechnology. In this case, it involves a fixed bed of microorganisms that degrade organic waste products until acceptable levels are achieved in the water to be discharged directly. The sludge from these purifiers is used as biomass for animal feed. There are also biotechnological processes for treating urban solid waste using aerobic or anaerobic fermentations to produce biogas. Another example of this technique includes testing treatments for point-source problems using biotechnology, such as the digestion of oil spills in the ocean by microorganisms following a tanker accident that led to a discharge.
2.3.2. Decontamination (Water, Air, Soil)
Currently, biotechnologies are primarily used to combat pollution. One of the first applications was the treatment of wastewater, followed by air purification and gaseous effluents. Biological decontamination is increasingly focusing on soil.
2.3.2.1.Microbiological Decontamination of Water
The treatment of wastewater heavily relies on biotechnologies: biological treatment allows for a much more efficient cleaning of a wide range of effluents than physico-chemical methods and is particularly suitable for those containing the most common organic pollutants. In fact, its use for wastewater treatment dates back over a hundred years.
Since then, both aerobic and anaerobic processes have been developed. Aerobic treatment has become the most common solution for lightly to moderately loaded effluents, as well as for toxic and recalcitrant molecules. Anaerobic processes are more effective for highly organic effluents, such as wastewater from food processing plants, urban sludge, and livestock waste, and have begun to replace aerobic systems in many applications over the past decade. Anaerobic treatment facilities are more compact, separating carbon compounds to produce combustible gas, methane, and offering recovery rates exceeding 80%. Biotechnological methods are now widely used to extract nitrates, phosphates, heavy metal ions, chlorinated organic compounds, and toxic substances. While the initial goal of wastewater treatment was primarily to reduce organic matter in general, the neutralization of industrial pollutants is now increasingly important, which is why efforts are underway to develop biological processes for extracting specific pollutants.
2.3.2.2.Microbiological Decontamination of Air
The use of microorganisms in the treatment of gaseous pollution is a relatively recent approach that relies on their ability to utilize pollutants in their metabolism to produce the energy necessary for cellular development.
For a better understanding of biotechnologies applied to reducing atmospheric pollution, such as desulfurization and denitrification of flue gases, removal of toxic products like organochlorines, deodorization, and volatile organic compounds (VOCs), some concepts of microbial metabolism are presented in this section. The use of microorganisms in biological treatments relies on their ability to utilize certain undesirable molecules for humans and the environment as substrates. Indeed, microorganisms, particularly bacteria, exhibit several capabilities, including:
- Rapid propagation with relatively short generation times.
- Significant flexibility in regulating, coordinating, inducing, and repressing metabolic pathways.
- Quick colonization of new habitats.
- Tolerance to extreme environmental conditions.
- Association with
other organisms in synergistic interactions such as symbiosis, mutualism,
and commensalism, thereby expanding the metabolic diversity of a species.
Biotechnological processes used for air and gaseous effluent treatment primarily include: - Biofilters,
- Biowashers,
- Percolation filters.
2.3.2.3. Microbiological Decontamination of Soils
Numerous companies specialize in soil decontamination. Although treatment methods may differ, the methodological approach remains consistent.
The first step: involves assessing the "damage." What is the extent of the pollution? Is it superficial or deep? What are the immediate dangers? What are the geological and hydrological data of the site?
The second step: is critical. Laboratory analysis allows for the examination of the nature of the pollution. Simultaneously, "microorganisms" are isolated from the contaminated site and tested to determine their biodegradative capacity. In some cases, at sites being rehabilitated, microorganisms have already initiated degradation. The isolation of these strains and the characterization of their nutritional requirements will help determine which nutrients need to be added to accelerate their development.
The speed and degree of pollutant degradation depend on numerous parameters that must be optimized, including:
- The concentration and nature of the pollutants,
- Interactions between different pollutants,
- Nutrients (sources of nitrogen, phosphate, minerals),
- Electron acceptors (oxygen, nitrate, etc.),
- Moisture (water activity plays a crucial role in biodegradation),
- Temperature,
- Soil structure.
The third step: specifies the technique. Should biodegradation occur "in situ," "on site," or off-site?
In situ Treatment: This is the preferred technology:
- When contamination is shallow, the soil can be enriched with microorganisms and nutrients, potentially tilled mechanically to promote oxygen transfer; the water resulting from the treatment is purified in a bioreactor.
- When the soil to be decontaminated is not accessible (occupied by structures) or when contamination is very deep and has already reached the groundwater.
In "in situ" treatments, the nature of the electron acceptor plays an important role. Oxygen, hydrogen peroxide, or nitrates can be used.On-site Treatment: After excavation, the soil is treated on-site using appropriate technologies:
- In bioreactors, - In windrows, - In piles.
To achieve this, the contaminated soil is homogenized, enriched with products that improve its structure (such as shredded straw); the soil is watered, enriched with nutrients and microorganisms (if applicable), and shaped into either windrows or piles.
The soil is regularly turned to ensure good oxygen transfer. As temperature plays animportant role, efforts are made to control it as much as possible (with piles or windrows being covered).
Off-site Treatment: Several industries offer to treat polluted soils at "waste treatment centers" using techniques involving more efficient reactors, sometimes combining soil, water, and gas treatment.
Transportation costs are high, but the efficiency of the equipment can sometimes accelerate the biodegradation process and thus reduce treatment costs.The fourth step: involves monitoring the process, which typically takes several months (in situ or on-site). Analytical monitoring allows for the assessment of:
- The disappearance of pollutants,
- The possible emergence of new molecules,
- Biodegradation kinetics to verify the performance of strains concerning their biodegradative capacities (evaluated in the laboratory) in order to adjust nutrient inputs if necessary.
Chapter 03: Biotechnology in Agronomy for Food Purposes
Chapter 03
3. Biotechnology in Agronomy for Food Purposes
3.1. Biotransformation and Preservation
3.1.1. Biotransformation
Fermentation is a process in which a food is transformed by microorganisms (biotransformation) in the absence of oxygen. These microorganisms are often referred to as “beneficial microbes.” For example, yogurt is the product of milk fermentation, and mold found on cheese, such as in Roquefort, is responsible for its characteristic green color and strong flavor. The process by which microorganisms and their enzymes induce these desirable changes in food is called fermentation. Humans use fermentation for three main purposes: to make food more digestible, to preserve it for longer periods, and to produce substances of interest.
Fermentation is also widely applied in the production of microbial cultures for enzymes, flavors, fragrances, food additives, and a range of other value-added products.
Microorganisms are used in the production of many products, such as:
- Beer, yogurt, Food additives
- Vaccines, antibiotics, antibodies, vitamins, amino acids
Which microorganisms are used in these processes? There are three main groups:
- Bacteria
- Yeasts
- Molds
3.1.2. Preservation
Biotechnology, as applied to food processing, relies on microbial inoculants that enhance properties such as flavor, aroma, shelf life, texture, and nutritional quality. Lactic acid bacteria are divided into two main groups based on the nature and concentration of the terminal products resulting from glucose fermentation: homofermentative and heterofermentative species. The main advantage of these bacteria lies in their ability to acidify food products. Lactic acid, as well as other organic acids (acetic acid, formic acid), are the fermentative metabolites and play a major role in food preservation by inhibiting the growth of pathogenic bacteria at low pH levels. Lactic fermentation is not only used for preserving dairy products but also allows for the preservation of various vegetables and fungi, including cabbage, beets, carrots, beans, onions, etc. This technique involves preserving vegetables by promoting the growth of lactic acid bacteria, which acidify the environment and thus inhibit the growth of undesirable organisms.
3.2. Production of Food Matrices in Bioreactors
A bioreactor, also known as a fermenter or propagator, is a device in which microorganisms (yeasts, bacteria, microscopic fungi, algae, animal and plant cells) are cultured for biomass production, metabolite production, or the bioconversion of a molecule of interest.
In the 1800s, Pasteur, Kutzing, Schwann, and Cagniard-Latour demonstrated that fermentation was caused by yeasts, which are living organisms. The term "fermentation" encompasses both aerobic and anaerobic metabolism. It involves multiplying the biomass of living microorganisms, and potentially utilizing their metabolism.
Unlike simpler systems used to grow microorganisms, such as flasks, a bioreactor allows for the control of culture conditions (temperature, pH, aeration, etc.), providing more reliable data. Laboratory models range from 0.1 to 15 liters. Models used for pilot-scale testing, aimed at industrialization, range from 20 to 1,000 liters, while those used for industrial production can exceed 1,000 cubic meters (as in ethanol production). Disposable bioreactor models have been available on the market since 1995, primarily used for volumes ranging from milliliters to several hundred liters.
In tissue engineering, the term "bioreactor" can refer to a system designed for tissue culture. Here, the goal is not to produce metabolites but to generate a complete tissue composed of cells and the extracellular matrix.
A Bioreactor consists of:
- A tank or chamber made of glass (for laboratory models) or stainless steel
- A cap, if necessary, to prevent the internal environment from being exposed to the external atmosphere
- A syringe with a catheter to inject a solution
- An agitation system featuring one or more impellers, depending on their size
- Sensors for measuring temperature (thermometer), pH (pH meter), dissolved oxygen concentration (oxygen probe), and level...
- A control system managed by a computer, allowing for the recording and regulation of all operational parameters
A fermenter is generally constructed based on the model of a bioreactor, but without an aeration system. In biotechnology, the term "fermenter" is sometimes used interchangeably with "bioreactor." It is used to distinguish between types of cultures (e.g., bacteria and yeast for fermenters, animal cells for bioreactors).
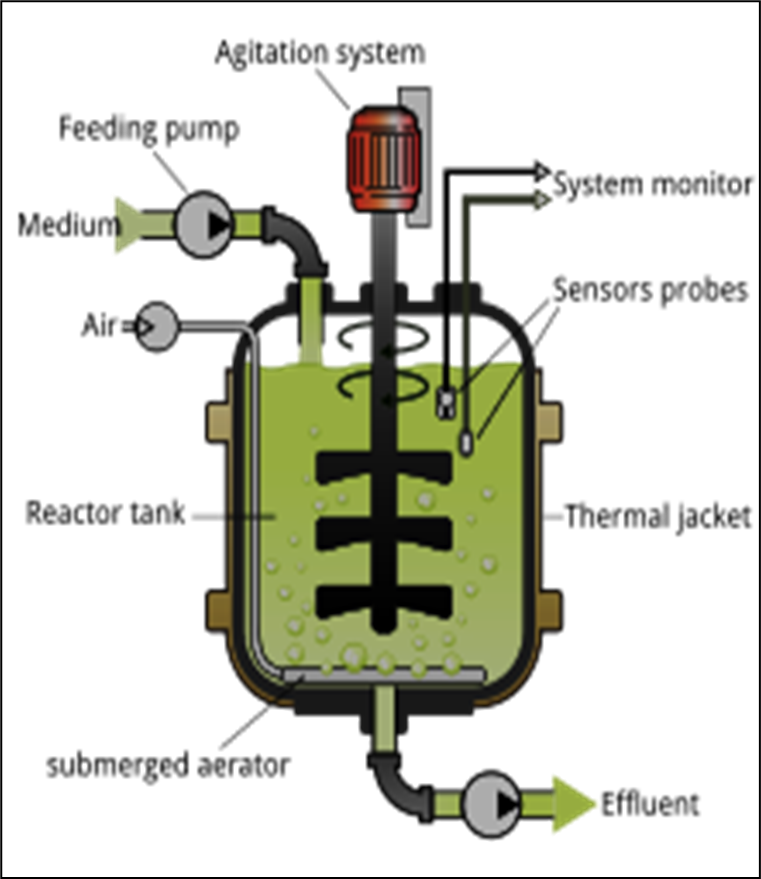
Fig: the components of a bioreactor
Components of a Bioreactor:
- Tank or chamber: Made of glass (for laboratory models) or stainless steel.
- Cap (if necessary): To prevent the exchange of air between the internal and external environments.
- Syringe with catheter: For injecting a solution.
- Agitation system: Consisting of one or more impellers, depending on their size.
- Sensors: For measuring temperature (thermometer), pH (pH meter), dissolved oxygen concentration (oxygen probe), and level…
- Control system: Managed by a computer, allowing the recording and control of all operational parameters.
Dairy Products
Milk:
Milk is composed of various substances, including fats, which are stabilized by a protein called casein, and carbohydrates, primarily in the form of lactose. The fermentation of milk allows for longer preservation and makes fermented dairy products more digestible. This transformation of milk is caused by the destabilization of casein micelles through proteolysis, resulting in coagulation.
In cheese-making, rennet technology is also used. In this case, coagulation of the casein is carried out enzymatically, notably through the action of chymosin. This coagulation leads to various consequences: it modifies the texture, flavor, and quality of the milk. The pH also decreases, which helps limit the growth of undesirable bacteria. Meanwhile, the starter cultures multiply and produce compounds responsible for the organoleptic properties of fermented dairy products.
Yogurts:
Yogurt is the product of milk fermentation by two lactic acid bacteria, Streptococcus thermophilus in symbiosis with Lactobacillus bulgaricus. The name "yogurt" is reserved for milk fermented by these two bacterial strains.
Yogurt production occurs in several stages. First, the milk is pasteurized, which eliminates pathogenic microorganisms. It is then inoculated after being cooled and maintained at a temperature of 43°C, the optimal growth temperature for lactic acid bacteria. The next step is incubation, where the milk is placed in containers for 3 hours, allowing the cultures to develop and transform the milk.
The bacteria present must still be alive at the time of yogurt consumption, which aids digestion and promotes better intestinal transit. The viable culture count at the time of sale must be greater than 107 germs/g of product.
Chapter 04: Biotechnologies and Industry for Non-Food Purposes
4.1. Bioenergy
Bioenergy refers to the forms of energy stored in biomass (mainly through the photosynthetic conversion of solar energy). This includes energy crops, agricultural and forestry residues, and organic waste, which can be used to produce heat, cooling, electricity, or fuels. As long as it is not overexploited, it is considered "renewable."
Fossil fuels (oil, natural gas, coal, etc.) were once thought to be inexhaustible. However, various oil crises have highlighted the importance of renewable energy as a substitute for petroleum products. One such energy source is biomass, often described as "the totality of all renewable raw materials of plant or animal origin intended for non-food uses."
Biomass is a diverse fuel. Broadly speaking, it includes "all living organisms, both animal and plant, as well as their products, by-products, or waste (excretions, etc.)." Biomass constitutes the various ecosystems of the planet and contributes to their natural balance. It was initially cultivated and raised by humans for food but also provides construction materials and serves as a raw material for certain industrial processes and energy production—referred to as bioenergy.
The difference between bioenergy and fossil fuels lies in their environmental impact: bioenergy can reduce greenhouse gas emissions. This is because the carbon released during fuel combustion can be recaptured by growing plants. However, the actual emissions reduction depends on the type of bioenergy production, the transformation process, and especially the location where the raw materials for bioenergy are produced. For instance, the quality of the fuel used is important (burning wet wood increases emissions two to four times compared to dry wood).
A renewable energy source is one generated from a resource that replenishes quickly enough to be considered inexhaustible. Fossil fuels (natural gas, oil, coal, etc.) took millions of years to form. Using these fuels much faster than they are formed depletes global natural reserves irreversibly for several generations. Fossil fuels are therefore not renewable energy sources.
Biomass, when cultivated or raised by humans, is expected to renew after each use. Thus, biomass is a renewable energy source, provided that agricultural and forestry systems are sustainable and responsibly managed.
4.1.1. Sources of Biomass:
Biomass is primarily composed of carbon, hydrogen, and oxygen, along with smaller amounts of nitrogen, derived from various types of resources:
- Agricultural products: These can be divided into:
- Traditional annual crop cultivation (e.g., cereals and oilseeds), mainly valued for their noble parts (grains, seeds, and tubers).
- Crops dedicated to biorefineries, as well as agricultural and livestock residues.
- Forestry products: These include logs, pellets, wood chips, and residues from forestry operations or specific silvicultural practices. The utilization of forest resources must comply with sustainable exploitation practices.
- Aquatic products: These include algae, fishing residues, and aquaculture by-products.
- Other organic waste: Examples include urban waste, sludge from wastewater treatment plants, household waste, and green waste from parks and gardens.
4.1.2.Applications :
- Wood heating: Using logs for individual heating systems.
- Collective wood boilers: These utilize sawmill waste (sawdust, wood scraps) or wood chips from forestry operations.
- Wood refining: Through dry distillation of wood, methanol can be obtained. Various processes exist for producing bioethanol from wood cellulose.
- Synthetic natural gas production: An innovative and promising method involves producing synthetic natural gas from wood, which can substitute or blend with fossil natural gas.
- The paper industry: It uses black liquor (a by-product of pulp production) with an energy content comparable to wood to produce steam and electricity through cogeneration.
A biofuel or agrofuel is a fuel (a liquid or gaseous biofuel) produced from non-fossil organic materials derived from biomass (hence the prefix "bio" in biofuel) and serves as a complement to or substitute for fossil fuels. Currently, two main production pathways exist:
- Oil-based and derivative pathway: Includes vegetable oil fuels, biodiesel (or biogasoline), as well as animal fats or various fatty acids (e.g., algae).
- Alcohol-based pathway: Includes bioethanol, derived from sugars, starch, cellulose, or hydrolyzed lignin.
Biogas is the gas produced by the fermentation of organic matter in the absence of oxygen. It is the result of the methanation or anaerobic digestion of fermentable waste. The most common sources of biogas come from voluntary or involuntary organic matter stocks:
- Crops: Cultivated specifically for energy purposes.
- Landfills: The biogas content varies depending on the operational methods and their sealing. Selective collection of organic waste enables faster methanation in landfills using specific bioreactors (digesters).
- Wastewater treatment plant sludge: Methanation eliminates organic compounds and allows treatment plants to achieve partial or full energy self-sufficiency.
- Livestock effluents: Regulations mandate storage facilities for effluents (slurry, manure) with a capacity of more than six months. This storage period can be used for the methanation of effluents.
- Agri-food industry effluents: These can also be methanized, primarily to prevent the discharge of overly rich organic matter and, in some cases, to enable energy recovery.
4.2. Biomaterials and Agro-Polymers
4.2.1. Biodegradable materials are materials capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass through the enzymatic action of microorganisms. The biodegradability of a material is thus defined as its intrinsic ability to be broken down by microbial activity, progressively simplifying its structure and ultimately converting it into CO₂, H₂O, and/or CH₄, as well as new biomass. Various sources of polymers can be used to produce such materials. Depending on the origin of the raw materials and the synthesis pathways, there are two main methods for producing biodegradable materials:
- Biodegradable polymers derived from the petrochemical industry.
- Biodegradable polymers derived from renewable resources.
The plastics industry is one of the most significant sectors within the chemical industry in terms of both quantity and diversity of applications. As this industry primarily relies on fossil resources, it must rapidly find alternatives to conventional raw materials. Due to their abundance and diversity, plant-based polymers offer a new source of renewable raw materials for the plastics industry.
The renewed interest in renewable raw materials within the plastics sector aligns with environmental sustainability goals and the need to manage finite fossil resources. Plant-based raw materials, primarily polymers, possess particularly attractive properties for the plastics industry, such as:
- Biodegradability.
- Biocompatibility.
- Selective permeability.
- Adjustable physico-mechanical properties.
These properties allow for targeted applications across various fields, including packaging, textiles, agriculture, pharmaceuticals, electronics, and medicine.
4.2.2.Major classes of bio-polymers derived from plants:
Polymers from plants, or bio-polymers (also called agro-polymers), often form the cell walls of plants, such as cellulose and lignin. Microorganisms can also produce polymers through the fermentation of plant-derived molecules, which are similarly classified as bio-polymers.
Polysaccharides (plants/algae) :
- Starch, Cellulose, Agar, Alginate, Carrageenan, Pectin, Gums, Konjac
Polysaccharides (via bacterial fermentation) :
- Xanthan, Dextran, Gellan, Curdlan, Pullulan, Elsinan
Proteins:
- Zein, Gluten, Polyamino acids
Polyphenols:
- Lignins, Tannins, Humic acids
Polyesters :
- Polylactic acid polymers (PLA)
- Polyhydroxyalkanoates (PHA)
Other polymers:
- Polymers synthesized from oil (e.g., nylon)
- Polyisoprenes: Rubber
4.2.3. The Biodegradability of Bio-Polymers
Bio-polymers are synthesized in plants or animals through enzymatic pathways, making them readily degradable in biological environments. The biodegradability of most bio-polymers is attributed to the presence of easily cleavable bonds, such as ester or amide bonds, which break down into simpler molecules and smaller fragments. These fragments can be assimilated by microorganisms for their biosynthesis, releasing CO₂ and H₂O.
In contrast, conventional petrochemical polymers, such as polyethylene or polypropylene, have a carbon backbone composed of strong covalent C-C bonds, requiring significantly more time and/or the presence of a catalyst (thermal, electromagnetic radiation, or chemical) for degradation.
4.2.4. Applications
Three main areas of application are identified based on the properties of bio-polymers:
- Medicine
- Agriculture
- Packaging
Bio-polymers are also used for more specialized and advanced applications in sectors such as the automotive industry, electronics, and construction.
4.3. Biomolecules and Cellular Activities
This field focuses on the production of products (polymers, sweeteners, amino acids, etc.), the invention of processes (biorefineries), or the generation of bioenergy on an industrial scale using biomass as a renewable raw material. These raw materials (e.g., corn, straw, sugar, beets, wood, oilseeds) are transformed into finished products (e.g., amino acids, enzymes, pharmaceuticals, ingredients, polymers, bioplastics, bioethanol), typically through the use of microorganisms. These methods represent the gradual transition of our industrial system from fossil primary raw materials to renewable biological materials.
White biotechnology is directly aligned with the goals of sustainable development by:
- Utilizing renewable carbon sources (instead of fossil carbon).
- Enabling reactions at normal temperatures (reducing energy consumption).
- Producing limited waste.
- Avoiding the use of solvents.
- Often minimizing water consumption.
These approaches lead to the concept of "integrated biorefineries," where plant-based raw materials (whole plants, residues, microorganisms) are used to produce a range of intermediate or final products for diverse industrial sectors (food, biofuels, biomaterials, additives, pharmaceuticals, enzymology, etc.).
Chapter 05: Microbial Biotechnology and Infectious Diseases
Chapter 05
5.Microbial Biotechnology and Infectious Diseases
Microorganisms are etymologically "small organisms," meaning living beings so small that they can only be observed under a microscope. This term encompasses a wide variety of species, whether prokaryotic (bacteria) or eukaryotic (yeasts, algae). Some also include viruses. Microorganisms represent the largest biomass on Earth.
We can define the fields of microbial biotechnology based on the products obtained:
• Production of microbial biomass for animal feed.• Microbial production of chemicals such as citric acid, glutamic acid, amino acids, etc.
• Microbial or enzymatic production of antibiotics and vitamins.• Production, from animal or plant cells or genetically modified microorganisms, of antigens, antibodies, therapeutic agents, and diagnostics previously produced from higher organisms.
5.1. Diagnostics
The healthcare sector (both human and veterinary) is increasingly relying on biotechnology to discover, test, and produce new treatments (e.g., vaccines, recombinant proteins, monoclonal antibodies, non-viral cell and gene therapy), but also to diagnose and understand the causes of diseases.
Most scientists working in the medical field tend to view biotechnology advancements as part of a continuum, represented by the ongoing process of refining and developing medical practices. Among the medical techniques, we have vaccination, veterinary diagnostics and treatments, artificial insemination, and genetic crossbreeding. Hundreds of tests used in medical diagnostics have been established. This includes the detection of the HIV virus and other diseases in their initial phase, allowing for the application of appropriate treatment. Home pregnancy tests without medical assistance are also biotechnology products.Applied Biotechnology in Analysis and Diagnostics has enabled the development of technologies and systems for detecting chemical or biological substances and microorganisms for their application in the characterization of industrial products, food, effluents, etc.
The discovery and utilization of microorganisms in medicine has led to revolutionary successes in the field of drug treatment. The first signs of this evolution were the discovery of penicillin, a product of a mold with antimicrobial properties, by Alexander Fleming in 1928/1929. Today, antibiotics account for about half of all drugs derived from biotechnology.5.2. New Therapeutic Pathways
Biotechnology applied to the pharmaceutical sector encompasses all techniques that use living resources to design and produce active substances.
Associated with genetic engineering, genomics is the foundation of current research in biotechnology. It contributes to new, more rational therapeutic approaches. Indeed, understanding genes and their products, proteins, allows us to understand their roles in a given disease and to define new molecular targets for developing an appropriate treatment.
The genomics of Mycobacterium tuberculosis has been studied to identify new drug targets and develop new vaccines against tuberculosis.New vaccines derived from genetic engineering induce effective immune responses and avoid potential side effects; this is why vaccines against Human Immunodeficiency Virus type 1 (HIV1) have been tested in animals.
DNA vaccination (or genetic vaccine) is a new vaccine approach. It is based on introducing DNA into cellular tissues. After DNA administration, the antigen is expressed by the cells, inducing a specific immune response. Thus, two genetic vaccines against toxoplasmosis have recently been developed.
Gene therapy represents another area of research. This therapeutic approach uses genes as medicines, either to compensate for defects in a gene affected by genetic diseases or to modify cellular behavior in the case of other pathologies. It involves introducing a gene into the nucleus of a living cell to induce a therapeutic effect.
The "gene medicine" is introduced into the cell in a targeted manner using gene transfer systems called vectors:
- Viral vectors, attenuated and genetically modified viruses to include the therapeutic gene;
- Synthetic vectors, lipid compounds, polymers, DNA nanoparticles (resulting from recent advancements in nanobiotechnology);
- Bacterial vectors, recombinant bacteria capable of transferring large DNA fragments (especially in the intestinal mucosa).
Vaccines from Recombinant Bacteria
- Vaccination with the recombinant Mycobacterium microti vaccine increases protection against tuberculosis. This vaccine induces the immune response of T lymphocytes.
- Ten recombinant proteins derived from Plasmodium falciparum, the parasite responsible for malaria, are produced by Escherichia coli. These proteins generate antibodies in rats.
- Brucella abortus is a Gram-negative intracellular pathogenic bacterium that infects animals or humans through the digestive tract. An antigen from Brucella abortus is produced in a recombinant Lactococcus lactis bacterium. This is the first step toward the development of live vaccines against brucellosis that can be administered orally.
- A study is underway on cancer vaccination trials. A recombinant protein consisting of an enzyme and an antigen associated with tumors has been expressed by a recombinant Escherichia coli strain. When injected into tumor-bearing mice, the recombinant protein shows anti-tumor activity.
- Recent research focuses on using recombinant bacteria as vectors for therapeutic genes.
- Work has been done to transfer a therapeutic gene into the intestinal mucosa by oral administration of a genetically modified, non-pathogenic, invasive Escherichia coli bacterium. A similar study aims to develop a recombinant Escherichia coli strain as a gene transfer vector in epithelial cells of the respiratory tract. These recombinant bacteria are capable of transferring large DNA fragments and open new perspectives for gene therapy.
Vaccines from Recombinant Viruses
- Research on vaccines against Human Immunodeficiency Virus type 1 (HIV1) is ongoing: recombinant attenuated measles viruses expressing HIV1 antigens have been developed. The immunogenicity of these recombinant vaccines has been tested in animals. The goal of this research is to develop an effective pediatric vaccine simultaneously against measles and AIDS.
- The recombinant Herpes Simplex Virus type 1 (HSV1) is used as a vector for vaccines. However, the immune response after vaccination is diminished in case of HSV1 seropositivity.
- A recombinant virus expressing a stimulating molecule infects dendritic cells and thus stimulates the immune system to counteract the growth of cancer cells. It can be used in cancer immunotherapy.