Detailed contents
This course is devided into four chapters, as represented in the mental map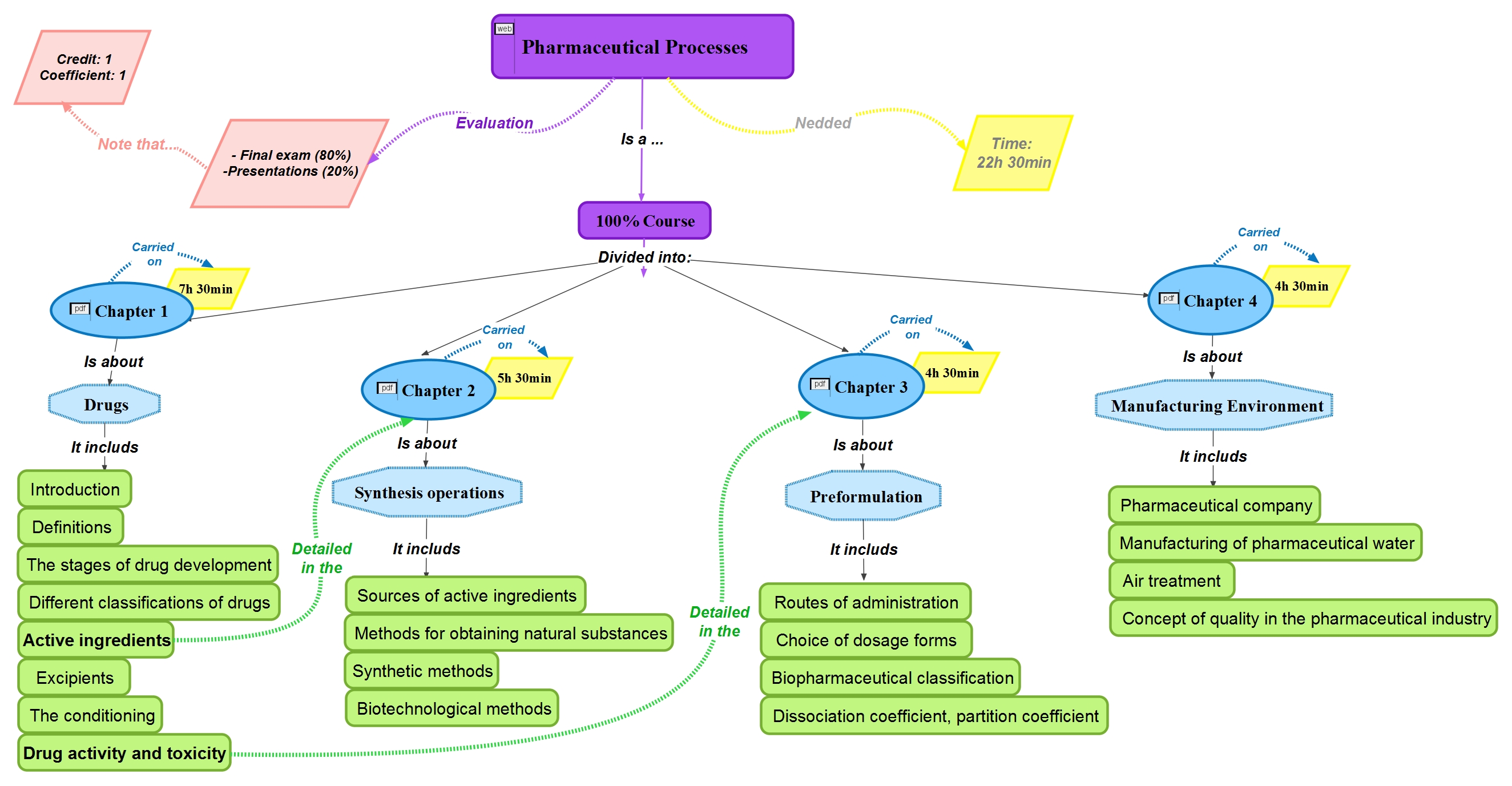
Chapter 1: The medicine
• Introduction
• Definitions
• The stages of drug development
• Different classifications of drugs
• Active ingredients
• Excipients
• The conditioning
• Drug activity and toxicity
• Become active ingredients in the body
Chapter 2: synthesis operations
• Sources of active ingredients
• Methods for obtaining natural substances
• Synthetic methods
• Biotechnological methods
Chapter 3: Preformulation
• Routes of administration
• Choice of dosage forms
• Biopharmaceutical classification (solubility, permeability)
• Dissociation coefficient, partition coefficient
Chapter 4: Manufacturing Environment
• Pharmaceutical company
• Manufacturing of pharmaceutical water
• Air treatment
• Concept of quality in the pharmaceutical industry
