-
Cacher les blocs
- Plein écran
- Vue standard
Aperçu des sections
Contact form
Institute : Science and Technology
Department: Common Core Department of Science and Technology.
Target public: First year common core GC, GM, GP
Fields of Specialization: chemistry
Course title: chemistry elements
Credit: 7
Coefficient: 4
Duration: 15 weeks
Weekly timetable: 1h 30 min of course and 3 hours for tutorial group
Place of teaching: Lecture hall 3 classroom 11
Teacher: Ikhlef Sofiane
Contact: s.ikhlef@centre-univ-mila.dz
Availability: every Tuesday from 9.30am-11am in office 19 (block 4).
Evaluation methods: A final exam 60%, short test 20%, practical work 20%.
Responses by e-mail: I promise to reply by e-mail within 24 hours of receiving the message, except in the case of unexpected circumstances.
Target skills
- Get to know some basic concepts in English.
- Learn about the history of discovering the components of the atom.
- Knowledge of the methods used to separate isotopes.
- To know the quantum numbers n, l, m and s of an electron.
- Write the electronic structures of atoms and position them in the periodic table.
Prerequisites

In order to achieve the objectives of this course, students must have certain prerequisites. They must know:
- Units of measurement.
- Physical constants.
- Some essential laws.
Chapter N° 1 : Basic concepts

From this chapter the learner will be able to:
- Describe chemical laws.
- Define the different types of matter.
- Knows the quantitative and qualitative aspects of matter
- Support in PDF

Chapter N° 2 : Composition of the Atom
Chapter N° 2 : Composition of the Atom
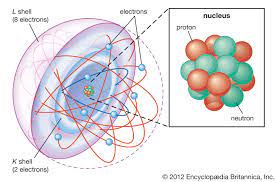
From this chapter the learner will be able to:
- describe the structure of an atom
- understand the use of the symbol X to describe a given atom.
- learn about the internal parts of an atom.
Support in Pdf

Chapter N° 3: Radioactivity
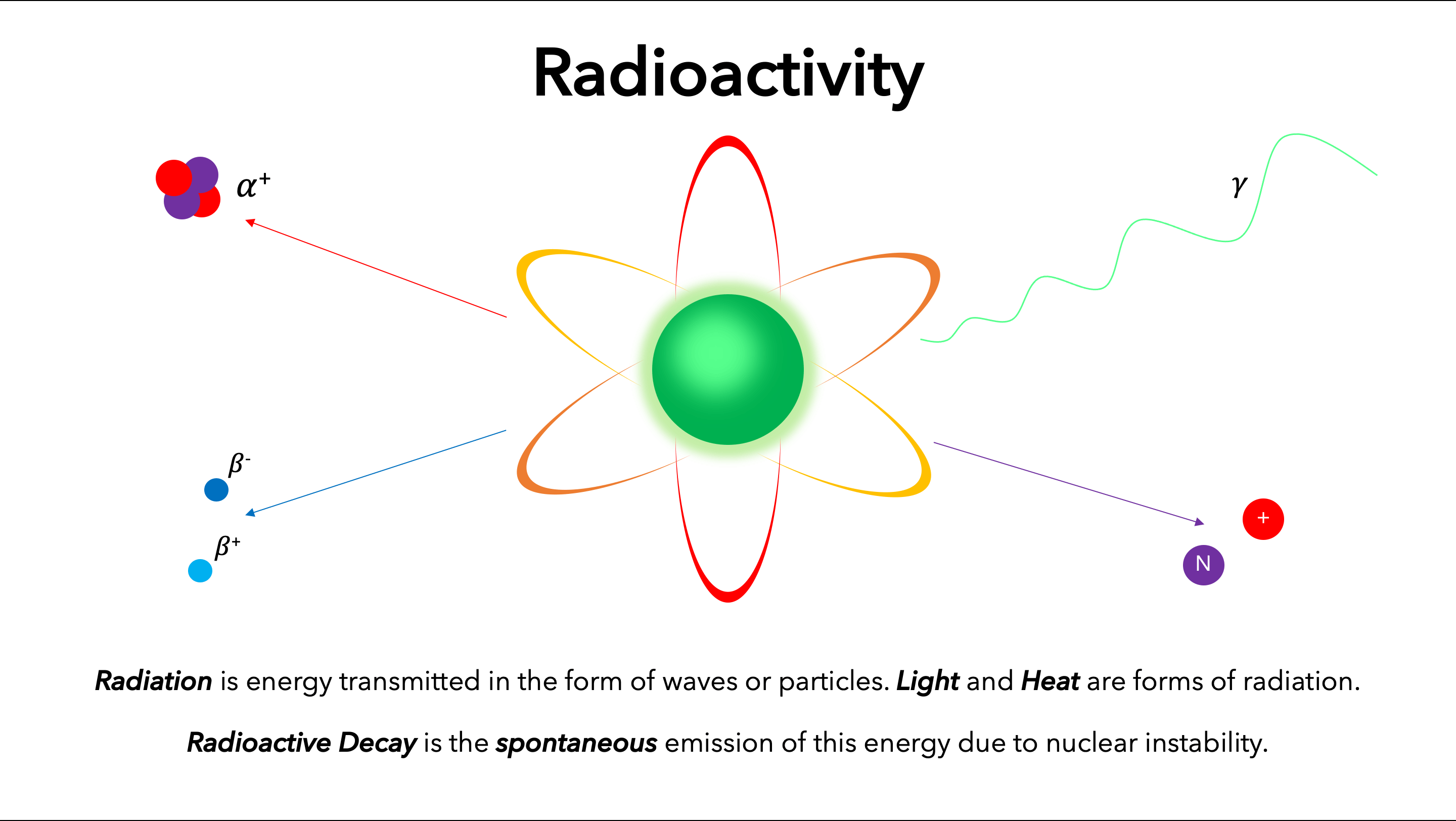
From this chapter the learner will be able to:
- understand half life
- describe nuclear equations for radioactive decay
- learn how one element may be changed to another by particule bombardment.
Support in PDF

Chapter N° 4 : Electronic structure of atoms
Chapter N° 4 : Electronic structure of atoms
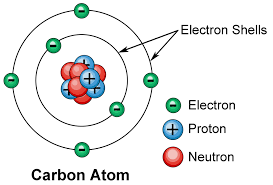
From this chapter the learner will be able to:
- describe the electronic structure of atoms
- arrange electrons inside an atom
- explain the electronic orbitals, Aufbau principale and Bohr theory.
Support in PDF

Chapter N° 5: Periodic Table of Elements
Chapter N° 5: Periodic Table of Elements
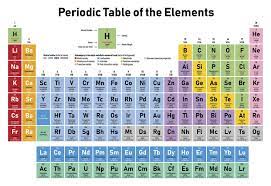
From this chapter the learner will be able to:
- identify groups and periods in the perdiodic table.
- know the positionns of groups
- predict the charge for element (ion) to reach maximum stability.
- understand how the periodic table was organized by mendeleev.
Support inPDF

References
References

1- James E. Brady, Johns R. Holum, Fundamentals of chemistry, third edition (john Wiley & Sons) (1988).
2- James E. Brady, Johns R. Holm, Fundamentals of chemistry, second edition (john Wiley & Sons. Inc) (1984).
3- Charles W. Keenan, Donald C. Kleifelter and jesse H. Wood, General college chemistry, sixth edition (Row, Publishers Inc. New York (1980).
4- James E. Brady and Gerard E. Humiston, General Chemistry, Principles and structure, (John Wiley & Sons) (1986).

